[Health Science domain]
Completion Of Human Milk Oligosaccharides (HMOs) Production Facility
- Products and Services
- Research and Technology
November 25, 2022
Kirin Holdings Company, Limited
Kyowa Hakko Bio Co., Ltd.
● Industrial-scale production of important ingredient for powdered infant formula
● Global market for HMOs CAGR expected to grow 20%-30% in the near future
● Sales to infant formula manufacturers and other customers to begin in 2023
TOKYO, November 25, 2022 – KYOWA HAKKO BIO CO. LTD. (Kyowa Hakko Bio), a subsidiary of Kirin Holdings Company, Limited (Kirin Holdings), completed a production facility for human milk oligosaccharides (HMOs) on November 18, 2022 at its Thai subsidiary, THAI KYOWA BIOTECHNOLOGIES CO., LTD. (Thai Kyowa).
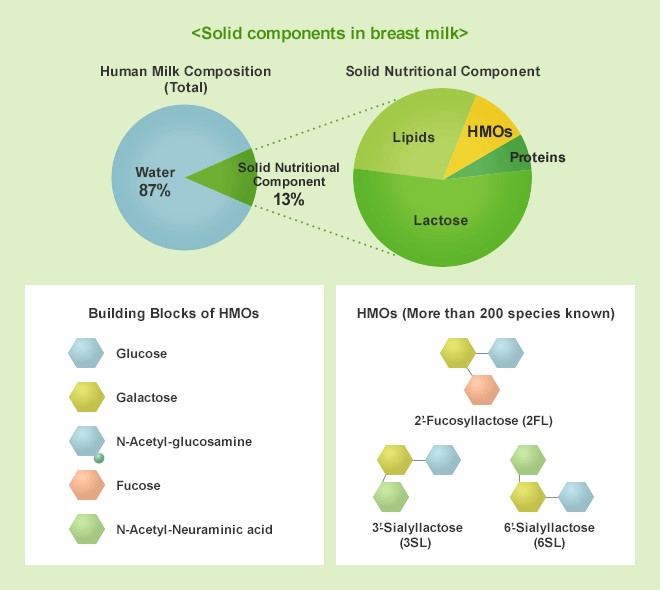
HMOs are oligosaccharides (complex sugar molecules)*3 found in breast milk. HMOs are the third most abundant solid component of breast milk after lactose and lipids, and more than 200 HMOs have been found in breast milk*1. Since HMOs are rarely found in cow's milk or milk of other mammalian origin, and are particularly abundant in human colostrum, they are known to be an important component for infants. Not only is the market for powdered infant formula containing HMOs continuously growing in Europe and the United States*2, but consumption is also expected to expand in China and Southeast Asia, where population growth is anticipated, with a projected CAGR of 20% to 30%*2 in the future.
*1: HMOs are composed mainly of glucose, galactose, fucose, N-acetylglucosamine, and N-acetylneuraminic acid.
*2: Barclays, "HMOs the next frontier of infant formula innovation," March 2022.
CAGR of the milk powder market with HMOs is projected for the period 2022-2027
In 2000, Kyowa Hakko Bio became the first company in the world*3 to establish an HMOs industrial-scale mass-production system. The production of HMOs will begin this year, with sales to infant formula manufacturers and other customers to begin in 2023. Based on the expansion into Asia, where consumption is expected to grow, and on the advantages of being able to secure excellent human resources and raw materials necessary for the production of HMOs, Kyowa Hakko Bio has built the Thai Kyowa production facility by integrating the knowledge and technology of the Kirin Group's engineering department. In addition to sales to infant formula manufacturers and other customers, products will be developed within the Kirin Group, aiming to expand its business to countries around the world where there is a high demand for HMOs.
*3: Tetsuo Endo et. al, Appl. Microbiol. Biotechnol. 53, 257-261 (2000)
The project has been granted investment incentive benefits by the Thai Board of Investment (BOI).
-

THAI KYOWA facility
-

Completion Ceremony (November 18, 2022)
Outline of the new HMOs Production Facility
| 1. Type of facilities | HMOs production facilities (fermentation, purification, and filling processes) |
|---|---|
| 2. Start of construction | November 2020 |
| 3. Products to be manufactured: | Three types of HMOs (2’-FL, 3’-SL, 6’-SL)* *: 2’-FL (2'-fucosyllactose), 3’-SL (3'-sialyllactose), 6’-SL (6'-sialyllactose) |
| 4. Production capacity | Approx. 300 metric tons/year |
| 5. Commercial production start | November 2022 |
Executive Comments on HMOs Production
-

Yuki Kanzaki, President & CEO, Kyowa Hakko Bio
Kirin Holdings has made a major shift to the Health Science domain (Health Science business), an area ranging from food to pharmaceuticals, in order to achieve the Kirin Group Vision 2027 (KV2027) long-term management plan. In this context, Kyowa Hakko Bio should play a central role in the Health Science business. The role of Thai Kyowa is particularly important, and this new facility is an indispensable production line to meet the global demand for HMOs. Through the production and sale of HMOs, Thai Kyowa will continue to contribute to solving the health issues of infants and young children.
-

Hiroshi Nagano, President, Thai Kyowa
We believe that HMOs are an important breast milk component for infants and are a material that can help people's health and quality of life. In order to contribute to the healthy development and growth of infants around the world, we at Thai Kyowa are committed to the safe and stable production of high-quality HMOs. We will also contribute to regional revitalization by creating jobs in Thailand, and aim to be a bridge between Thailand and Japan.
About Human Milk Oligosaccharides
“HMOs” (Human Milk Oligosaccharides) is the generic name for oligosaccharides*4 (complex sugar molecules) found in breast milk, and is the third most abundant solid component of breast milk after lactose and lipids. To date, more than 200 HMOs have been discovered in breast milk.
HMOs are known to be an important component for infants because they are rarely found in cow's milk or milk derived from other mammals and is especially abundant in human colostrum.
*4: Oligosaccharides: sugars consisting of several monosaccharides joined together; HMOs are mainly composed of glucose, galactose, fucose, N-acetylglucosamine, and N-acetylneuraminic acid.
HMOs Features
HMOs are prebiotics*, substances that serve as nutrients for beneficial bacteria such as bifidobacteria. They reach the large intestine without being metabolized by human digestive enzymes, are metabolized by intestinal bacteria, and exhibit various physiological functions. As research on HMOs continues, they are expected to function not only as an important breast milk ingredient for infants, but also as a "functional ingredient."
*Prebiotics: substances that serve as a selective source of nutrients for microorganisms beneficial to the human body and promote their growth and proliferation.
About Kirin Holdings
Kirin Holdings Company, Limited is an international company that operates in the Food & Beverages domain (Food & Beverages businesses), Pharmaceuticals domain (Pharmaceuticals businesses), and Health Science domain (Health Science business), both in Japan and across the globe.
Kirin Holdings can trace its roots to Japan Brewery which was established in 1885. Japan Brewery became Kirin Brewery in 1907. Since then, the company expanded its business with fermentation and biotechnology as its core technologies, and entered the pharmaceutical business in the 1980s, all of which continue to be global growth centers. In 2007, Kirin Holdings was established as a pure holding company and is currently focusing on boosting its Health Science domain.
Under the Kirin Group Vision 2027 (KV 2027), a long-term management plan launched in 2019, the Kirin Group aims to become A global leader in CSV*, creating value across our world of Food & Beverages to Pharmaceuticals. Going forward, the Kirin Group will continue to leverage its strengths to create both social and economic value through its businesses, with the aim of achieving sustainable growth in corporate value.
* Creating Shared Value: combined added value for consumers as well as for society at large.