[Soft drinks & Health science]
Lacticaseibacillus paracasei KW3110 Confirmed to Alleviate Mild to Moderate Hay Fever Symptoms
- Demonstrated Improvement in Nasal Symptoms and Outdoor Activity Limitations -
- CSV
- Research and Technology
October 31, 2025
Kirin Holdings Company, Limited
TOKYO, October 31, 2025 - Kirin Holdings Company, Limited (Kirin Holdings) has conducted a clinical trial to evaluate whether consumption of food containing Lacticaseibacillus paracasei KW3110 (hereinafter L. paracasei KW3110)*1 affected the nasal and ocular symptoms associated with hay fever seasonal allergy ranging from mild to moderate. The results of the trial confirmed relief of nasal symptoms, particularly during the “initial pollen dispersal period,” and showed a significant improvement in the degree of daily life impairment, specifically in “impairment of outdoor activities.” These research findings were presented at the 74th Annual Meeting of the Japanese Society of Allergology held at the Tokyo International Forum from Friday, October 24 to Sunday, October 26, 2025.
*1. International Archives of Allergy and Immunology 2004; 135:205–215.
According to a “Hay Fever Countermeasures Survey” conducted by Weathernews Inc., over half of the respondents reported having hay fever.*2 In recent years, symptoms such as sneezing, runny nose, and nasal congestion caused by hay fever (seasonal allergic rhinitis) have significantly disrupted daily life. The impact on quality of life (QOL) is particularly severe, including “impediments to outdoor activities,” “reduced concentration,” and “sleep disturbances.”*3 Hay fever is increasingly being recognized as a societal issue. Allergic reactions such as hay fever are closely associated with immune cells like Th2 cells, which trigger Type 2 inflammation*4. Allergic symptoms occur when these cells elicit excessive inflammatory responses to substances such as pollen, dust mites, and house dust, making it essential to prevent the exacerbation of Type 2 inflammation. Kirin has conducted experiments to compare the release levels of cytokines*5 that suppress Type 2 inflammation (e.g., IL-12*6). In these experiments, “L. paracasei KW3110” was compared with lactic acid bacteria derived from commercially available yogurt. The results confirmed that “L. paracasei KW3110” has a superior ability to induce the production of cytokines that suppress Type 2 inflammation compared to other lactic acid bacteria (Figure 1).
-
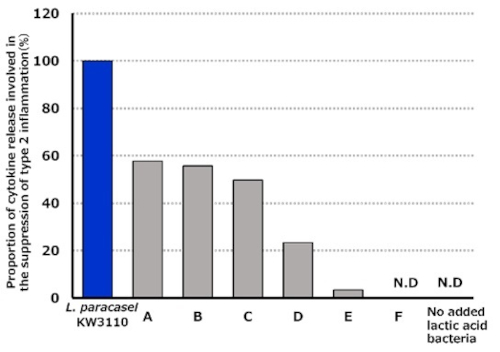
Figure 1. Proportion of type 2 inflammation-suppressing cytokines released by immune cells stimulated with various lactic acid bacteria
This graph is based on the content of the paper referred to in footnote 1.
Based on these foundational research findings, we initiated this clinical trial to explore the further potential of L. paracasei KW3110. We believe that the insights gained from this study will be meaningful in addressing health challenges faced by individuals with reduced quality of life (QOL) due to mild to moderate hay fever, through food-based solutions.
*2 Weathernews Inc. Hay Fever Countermeasures Survey https://jp.weathernews.com/news/45731/
*3 Ministry of Health, Labour and Welfare For Appropriate Hay Fever Treatment(https://www.mhlw.go.jp/new-info/kobetu/kenkou/ryumachi/dl/kafun_chiryo.pdfPDF:767KB)
*4 A type of inflammation associated with allergic reactions.
*5 A type of signaling molecule involved in allergic reactions.
*6 A cytokine that suppresses allergic reactions associated with type 2 inflammation. Figure 1 shows the production levels of IL-12.
Research Findings (Overview)
A clinical trial was conducted involving 120 men and women aged from 20 to 64 with mild to moderate allergic rhinitis to evaluate the effects of L. paracasei KW3110 on nasal and ocular symptoms as well as quality of life (QOL). During the spring pollen season (January to April), participants consumed either a food containing L. paracasei KW3110 (50 mg heat-killed bacteria, equivalent to over 140 billion cells) or a placebo food (without L. paracasei KW3110) for 12 weeks. The group that consumed the L. paracasei KW3110-containing food showed improved nasal symptom scores and reduced impairment in outdoor activities compared to the placebo food group, particularly during the initial pollen dispersal period observed at week 4 of consumption (Fig. 2).
-
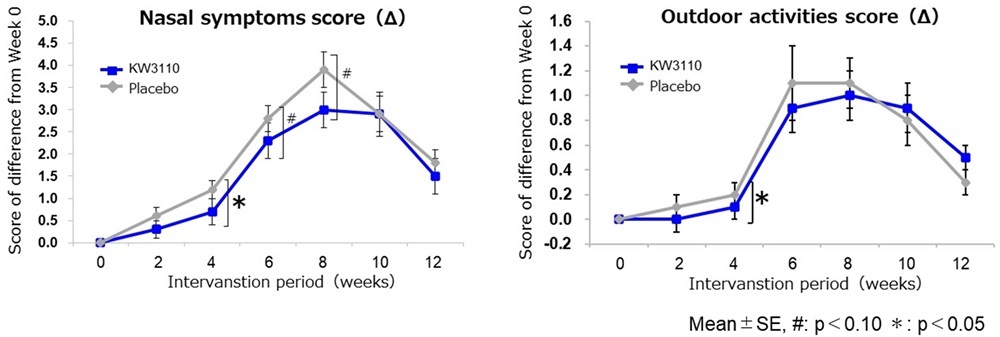
Figure 2. Evaluation of nasal symptom scores and outdoor activity scores
Implications
The research results indicate that L. paracasei KW3110 helps reduce nasal discomfort during the early pollen dispersal period and alleviates the decline in quality of life (QOL) associated with limitations in outdoor activities.
Future Prospects
Through continued research on L. paracasei KW3110, we aim to contribute to addressing health challenges such as hay fever by alleviating the decline in quality of life (QOL) caused by allergy symptoms.
Expert Commentary Provided by Dr Yoshitaka Okamoto, MD, PhD
According to a survey conducted by Japan’s Ministry of Health, Labour and Welfare, approximately one in two people in Japan currently suffer from some form of allergic disease, and the number of allergy sufferers continues to rise each year. Among these, allergic rhinitis affects nearly half the population. Cedar pollen allergy, in particular, is referred to as a “national disease” due to its high prevalence and severity of symptoms, posing a significant societal challenge.
Our research group has previously published findings indicating that L. paracasei KW3110 may help improve allergy symptoms in individuals with hay fever. The results of this clinical trial suggest that consuming this strain as a food may improve quality of life, particularly for individuals with mild nasal allergy symptoms. Naturally, individuals with severe symptoms should seek medical attention. However, the safety and convenience of consuming lactic acid bacteria as a food are noteworthy. Among lactic acid bacteria, L. paracasei KW3110 is readily taken up by immune cells, where it elicits a unique response that may help suppress allergic reactions. We anticipate continued research to further elucidate the distinctive functions of this specific strain.
Moving forward, we anticipate that further elucidation of its mechanisms of action, along with the accumulation of new clinical data, will lead to potential improvements not only in rhinitis symptoms such as hay fever, but also in a broader range of allergy-related conditions.
Dr Yoshitaka Okamoto, MD, PhD
Director, Chiba Rosai Hospital, Japan Organization of Occupational Health and Safety (JOHAS)
Professor Emeritus, Chiba University
Graduated from the Faculty of Medicine, Akita University in 1979. Conducted research in mucosal immunology as a research fellow at the State University of New York at Buffalo. Served as Professor of Otolaryngology at Yamanashi Medical University, Professor of Otolaryngology and Head and Neck Oncology at Chiba University Graduate School, and Vice President of Chiba University Hospital before assuming his current position in 2019. Specializes in otolaryngology, with a focus on the treatment of head and neck tumors, as well as the research and management of upper airway immunology and allergies. Served as President of the 67th Annual Meeting of the Japanese Society of Allergology. Previously conducted research on Lacticaseibacillus paracasei KW3110 and its relationship to allergic conditions.
About Kirin Holdings
Kirin Holdings Company, Limited is an international company that operates in the Food & Beverages domain (Food & Beverages businesses), Pharmaceuticals domain (Pharmaceuticals businesses), and Health Science domain (Health Science business), both in Japan and across the globe.
Kirin Holdings can trace its roots to Japan Brewery, which was established in 1885. Japan Brewery became Kirin Brewery in 1907. Since then, the company has expanded its business with fermentation and biotechnology as its core technologies, and entered the pharmaceutical business in the 1980s, all of which continue to be global growth centers. In 2007, Kirin Holdings was established as a pure holding company and is currently focusing on boosting its Health Science domain.
Under the Kirin Group Vision 2027 (KV 2027), a long-term management plan launched in 2019, the Kirin Group aims to become “A global leader in CSV*, creating value across our world of Food & Beverages to Pharmaceuticals”. Going forward, the Kirin Group will continue to leverage its strengths to create both social and economic value through its businesses, with the aim of achieving sustainable growth in corporate value.
* Creating Shared Value. Combined added value for consumers and society at large.
Effects of Lacticaseibacillus paracasei KW3110 on Mild to Moderate Seasonal Allergic Rhinitis Symptoms
About Lacticaseibacillus paracasei KW3110 (L. paracasei KW3110)
Lacticaseibacillus paracasei KW3110 is a strain of lactic acid bacteria originally isolated from cheese. It was identified through joint research by Kirin and Koiwai Dairy, selected from over 100 strains. This strain is expected to help regulate immune balance.
Background and Purpose
Hay fever (seasonal allergic rhinitis) is an allergic disease triggered by pollen, most commonly presenting as allergic rhinitis (sneezing, runny nose, nasal congestion) and allergic conjunctivitis (itchy eyes, tearing). The prevalence of hay fever (seasonal allergic rhinitis) exceeds 40% of the population in Japan. Notably, the prevalence of cedar pollen allergy has increased by approximately 10% over the past decade, and further increases in hay fever prevalence are anticipated, presenting a significant societal challenge. There is a growing demand for the development of products that utilize beneficial components found in food and beverages to safely alleviate allergy symptoms, with minimal concerns such as drowsiness. Through previous research, Kirin has demonstrated that L. paracasei KW3110 activates macrophages, a type of innate immune cell, and enhances Th1 cytokine production, in addition to its effects in relieving allergy symptoms. This study aims to develop a product containing L. paracasei KW3110 to alleviate allergy symptoms such as hay fever (seasonal allergic rhinitis), with the goal of improving the quality of life (QOL) diminished by rhinitis symptoms.
Research Method
The study was designed as a randomized, double-blind, placebo-controlled trial. A total of 120 participants (men and women aged 20 to 64 years) who experienced nasal and ocular discomfort in early spring and did not regularly use medications for allergic rhinitis symptom relief were enrolled. Participants consumed either a food containing Lacticaseibacillus paracasei KW3110 (50 mg heat-killed bacteria, equivalent to at least 140 billion cells) or a placebo food for 12 weeks (January to April). Self-reported nasal and ocular symptoms were assessed using the Japanese Standard QOL Questionnaire for Allergic Rhinitis, and the severity of allergic rhinitis symptoms was evaluated based on an otorhinolaryngologist diagnosis.
Results
Compared to the placebo, consumption of food containing L. paracasei KW3110 significantly improved subjective symptom scores (“nasal symptoms” and “impairment in outdoor activities”) and severity classification scores (“sneezing attacks or rhinorrhea” and “severity”) during the early pollen season at week 4 of intake (Figs. 1 and 2).
-

Figure 1. Evaluation of nasal symptom scores and outdoor activity scores
-
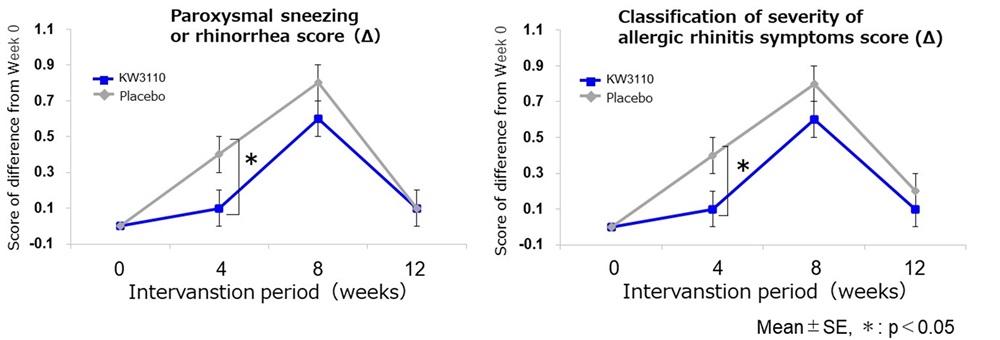
Figure 2. Classification of severity of allergic rhinitis symptoms by otorhinolaryngology
Research Findings
This study suggests that L. paracasei KW3110 reduces nasal discomfort during the early pollen dispersal period and mitigates the decline in quality of life (QOL) associated with difficulties in outdoor activities.
GALLERY
-
Proportion of type 2 inflammation-suppressing cytokines released by immune cells stimulated with various lactic acid bacteria
-

Evaluation of nasal symptom scores and outdoor activity scores
-
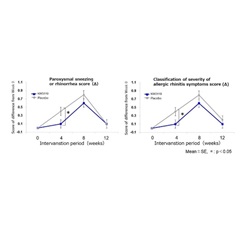
Classification of severity of allergic rhinitis symptoms by otorhinolaryngology
-

Dr Yoshitaka Okamoto, MD, PhD
